Features & Benefits
- Skin friendly NeoBond® Hydrocolloid tabs
- Macro and Jumbo sizes available with medical grade acrylic adhesive
- Designed to reduce extubations, help prevent palate trauma and allow for better oral care
- Eliminates need for tape near nose or mouth
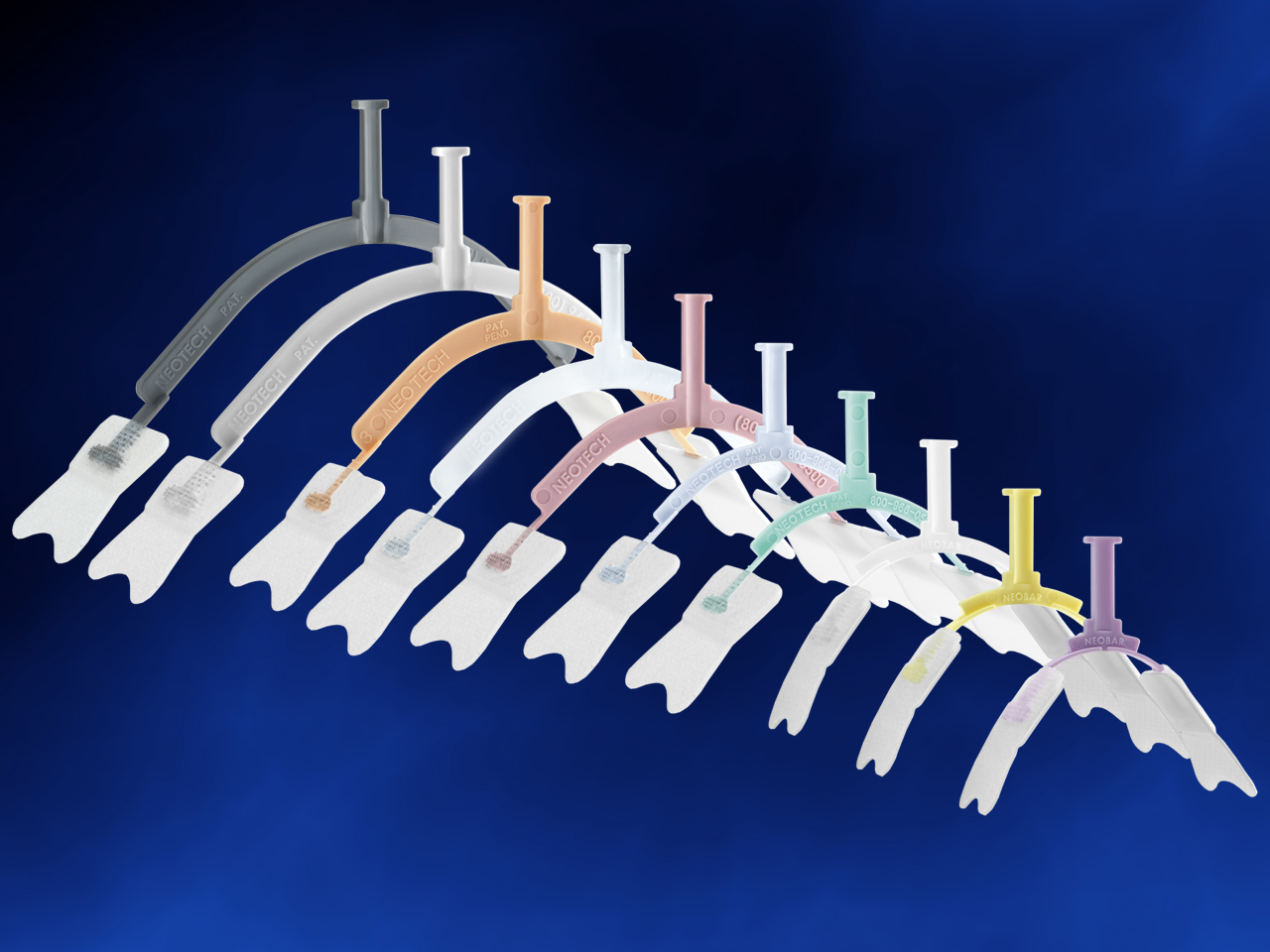
- Color coded sizes
- Not made with natural rubber latex or plasticizer DEHP
- Individually packaged
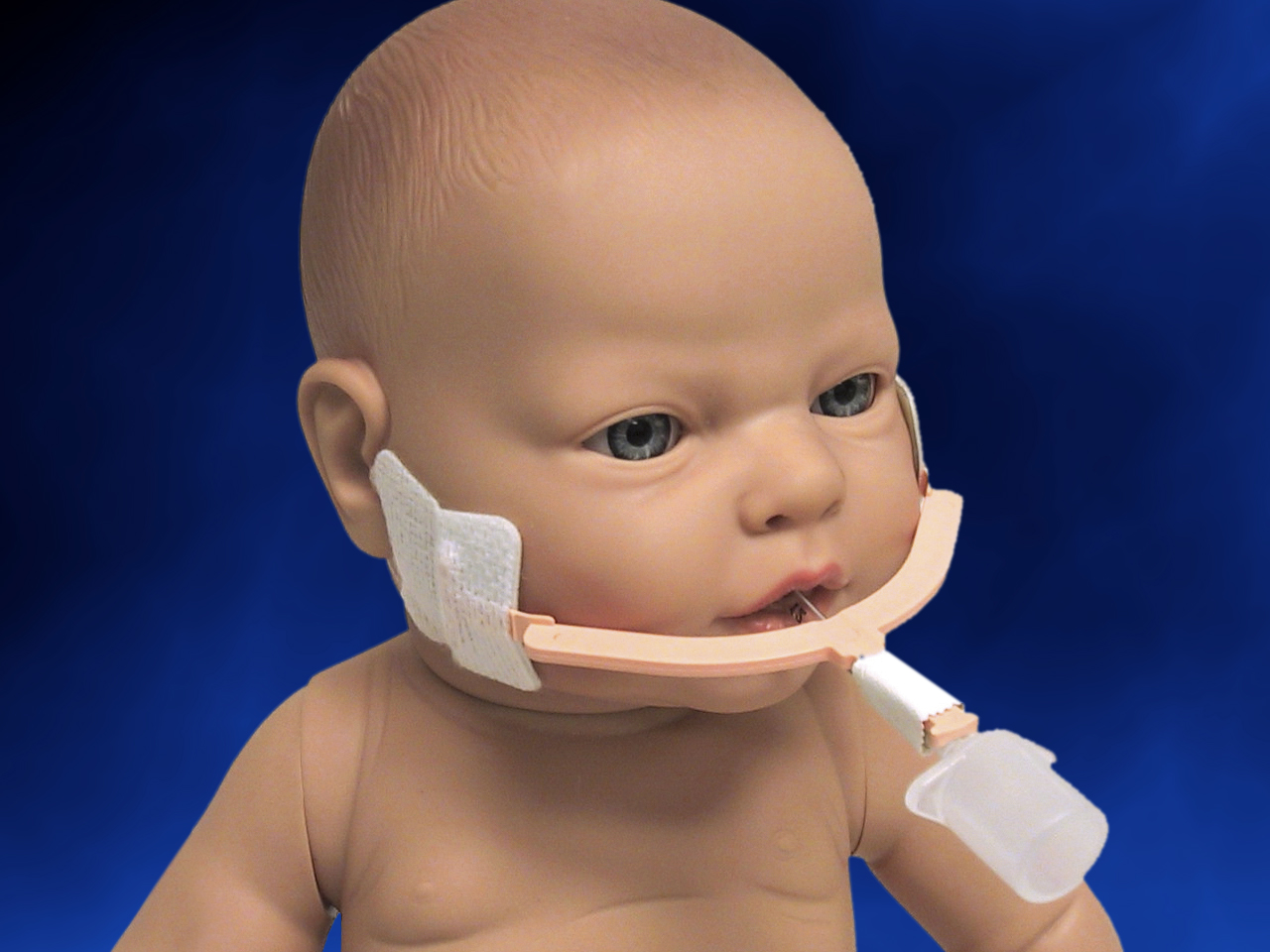
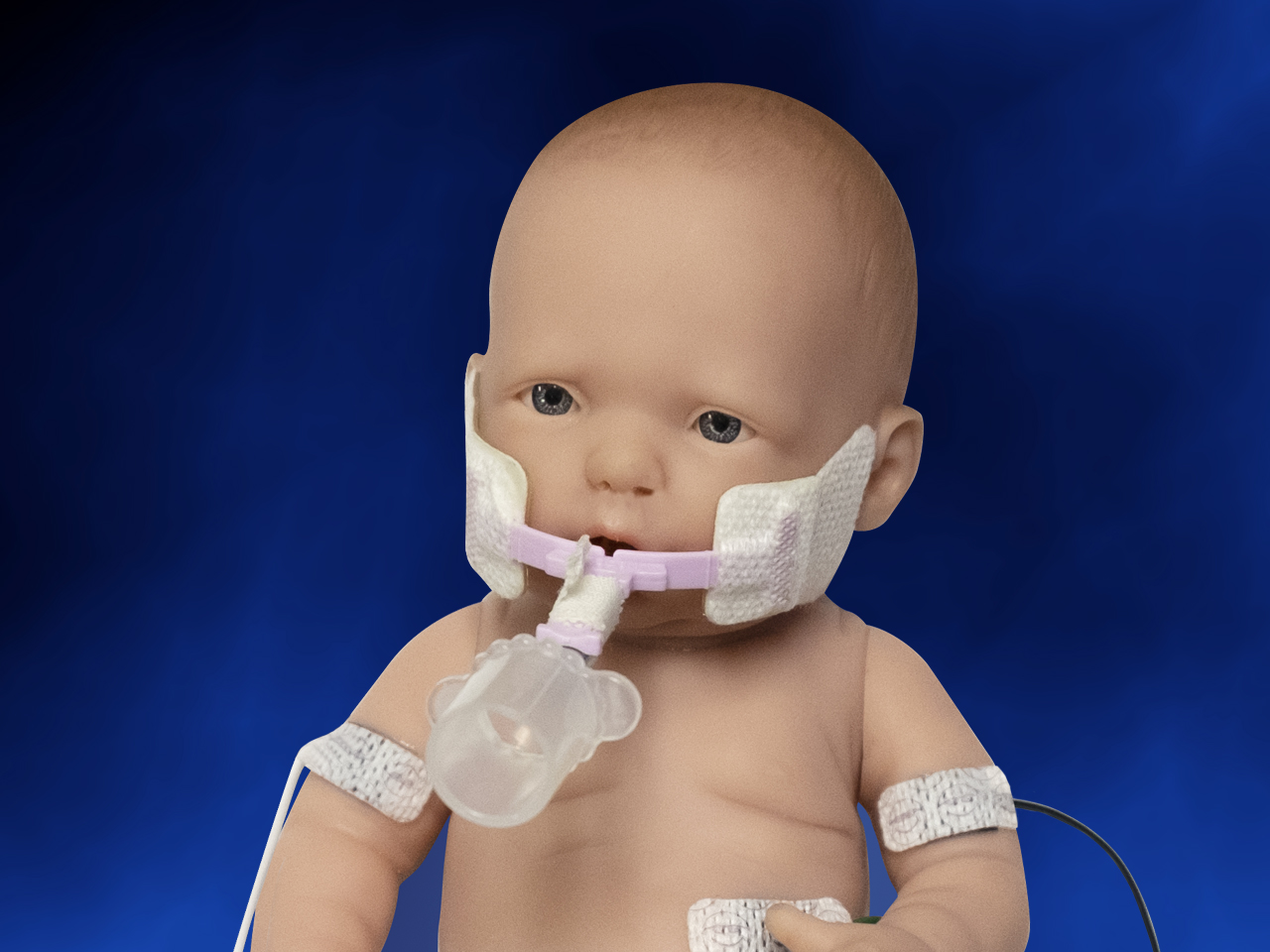
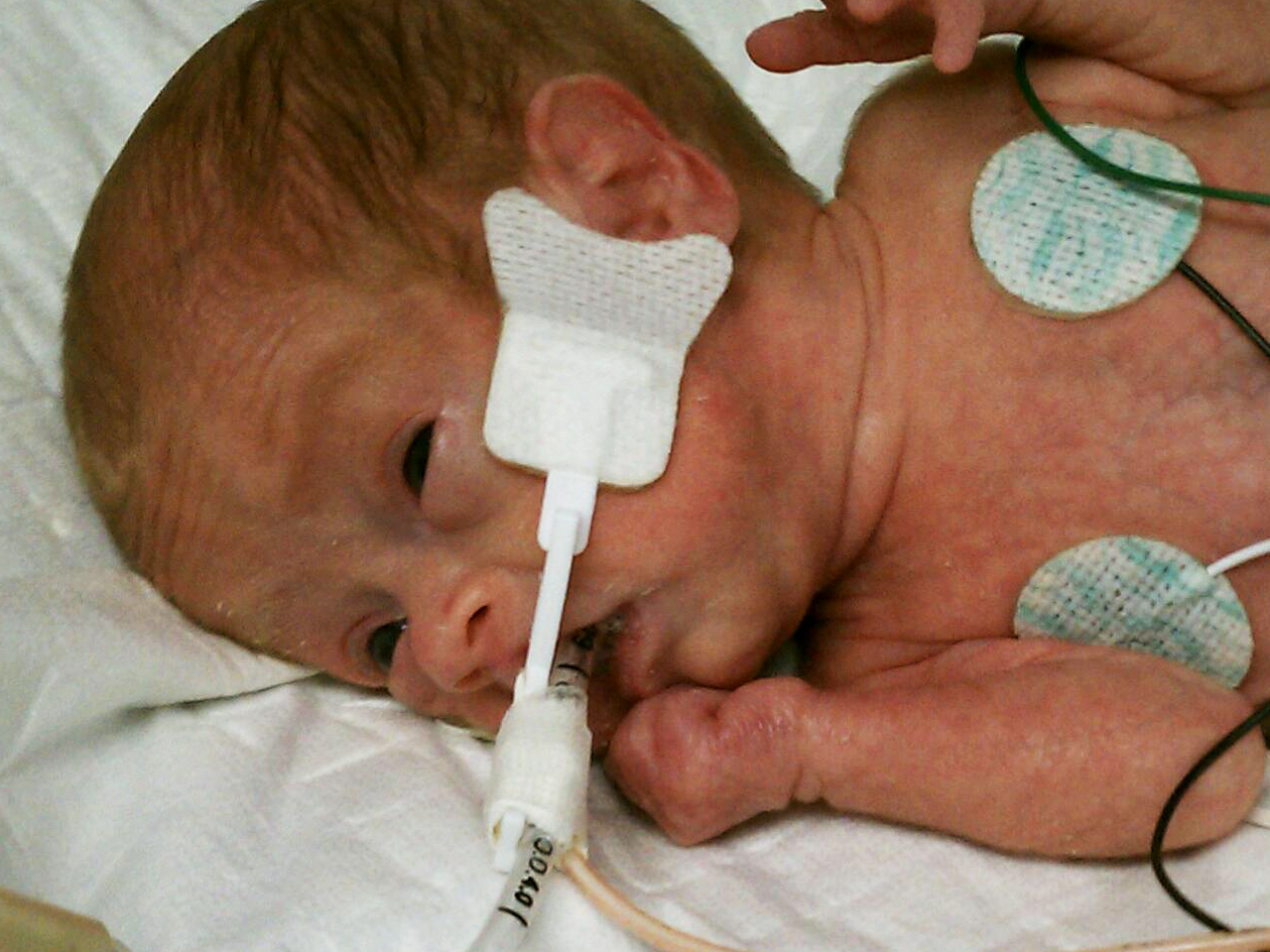
When you need to secure a baby’s endotracheal tube, Neotech’s NeoBar offers the ideal balance between security and comfort. The eight color-coded sizes make unit selection easier, and the skin friendly tabs are designed to reduce the use of potentially harmful tape on the skin. The NeoBar is designed to protect the patient’s palate and reduce the occurrence of extubations.
Directions for Use
Visit our YouTube channel for more DFU videos and languages.
Preparation
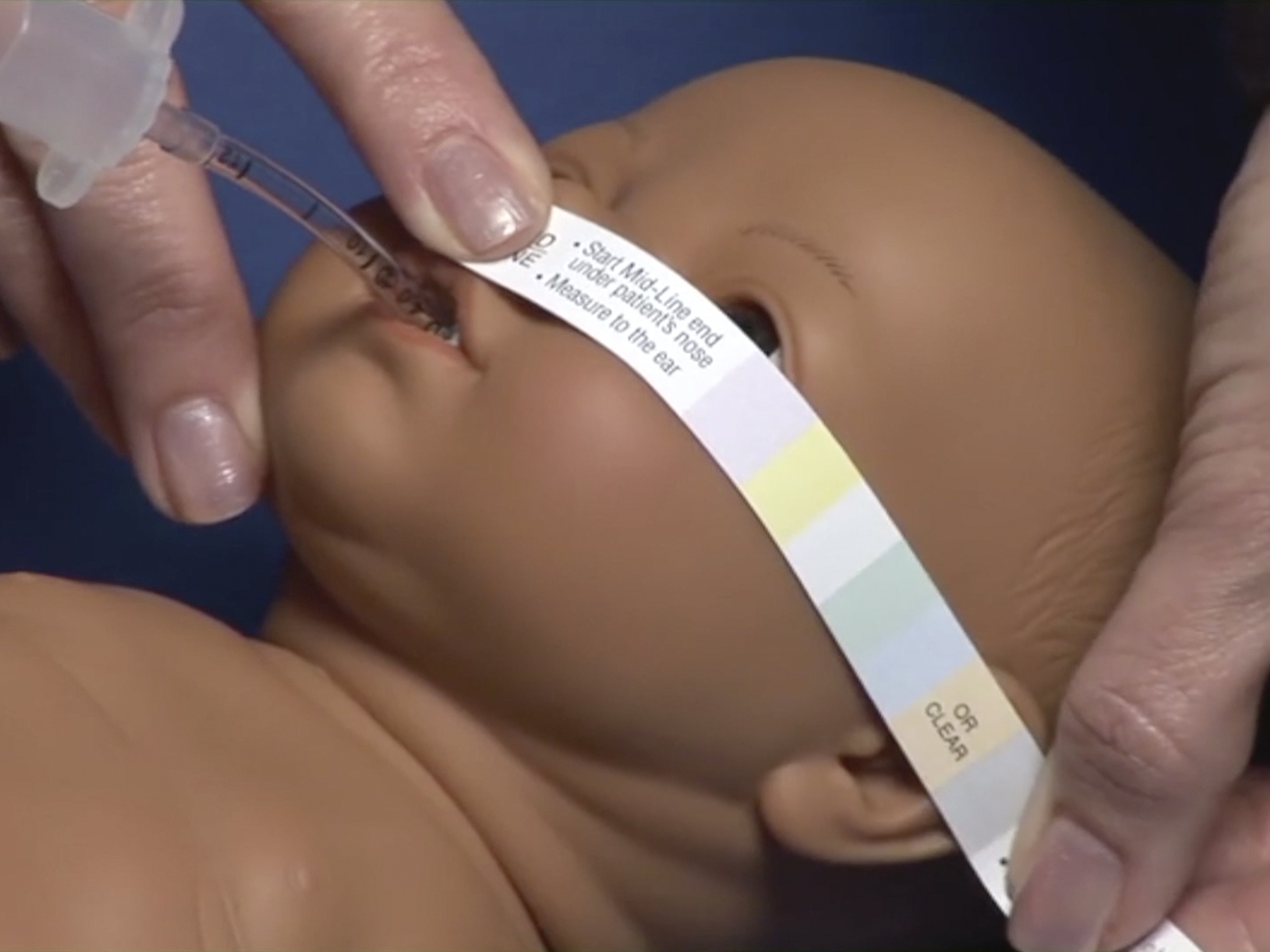
Step 1 After patient is intubated, select proper size NeoBar by using NeoBar measuring tape strip provided.
Place mid line of strip at nasal septum and wrap to ear. Color that falls over opening of ear canal corresponds to correct NeoBar size.
Note: if unsure between two sizes, try smaller size first.
Step 2 To ensure proper fit, position NeoBar across center of mouth, between upper and lower lips. NeoBar should not contact lips. Hydrocolloid tabs must be just in front of ear.
Step 3 Clean and dry skin on cheeks per hospital protocol.
Note: It is important for skin to be as clean and dry as possible. Hydrocolloid adhesive tabs will not adhere properly to moist skin or hair. Oils and lotions will adversely affect adhesion.
DO NOT USE ALCOHOL.
Application
Step 1 Before removing plastic liners, hydrocolloid ahesive tabs must be actively warmed. Warming techniques include: hold between the palms of your hands for at least 60 seconds, hold near a radiant warmer for 10-15 seconds, or hold against a chemical heel warmer for 10-15 seconds.
Step 2 Peel and discard plastic liners. Apply hydrocolloid adhesive tabs in front of ear on bone and hold in place for 60 seconds to ensure proper adhesion.
Note: Ensure ET tube is below platform to reduce pressure on palate or gums.
Step 3 Wrap 1/2″ (12.5 mm) white or cloth tape completely around NeoBar platform first, then continue taping around both platform and ET tube.
Removal
Step 1 Replace NeoBar every 5 days or per hospital protocol, whichever is sooner.
Step 2 Unwrap tape to separate ET tube from NeoBar platform.
Step 3 Saturate tabs with water or saline.
Step 4 Slowly peel hydrocolloid adhesive tabs away from skin as you swab with water or saline.
INDICATIONS FOR USE
The NeoBar is intended to secure an endotracheal tube. It is intended for use on pediatric (neonates, infants and children) patients.
CONTRAINDICATIONS, CAUTIONS & WARNINGS
The NeoBar is contraindicated for patients with infected or excoriated skin areas that would prevent the proper placement of the tabs.
It is not intended for use on adults.
Federal law restricts this device to sale by or on the order of a physician.
NeoBar should only be used while the patient is under the continuous, direct supervision of trained healthcare professionals.
Discontinue use immediately if skin irritation occurs.
NeoBar should not contact lips.
Hydrocolloid adhesive tabs will not adhere properly to moist skin or hair. Oils and lotions will adversely affect adhesion.
Do not use alcohol to clean skin prior to application.
Single patient use only.
Catalog
| Cat No. | Size | Color | Qty/Unit |
| N709 | Mini | Purple | 5/box |
| N710 | Ultra | Yellow | 5/box |
| N711 | Micro | White | 5/box |
| N712 | Small | Green | 5/box |
| N713 | Large | Blue | 5/box |
| N714 | XL | Rose | 5/box |
| N715H | Macro | Clear | 5/box |
| N716H | Jumbo | Gray | 5/box |
| With Medical Grade Acrylic Adhesive | |||
| N715F | Macro | Peach | 5/box |
| N716F | Jumbo | Carbon | 5/box |
| Transport Box | |||
| N718 | Multiple | Various | 6/box* |
| *One each: N709, N710, N711, N712, N713, N714 | |||
| Neonatal Assorted | |||
| N719N | Multiple | Various | 12/box* |
| *Two each: N709, N710, N711, N712, N713, N714 | |||